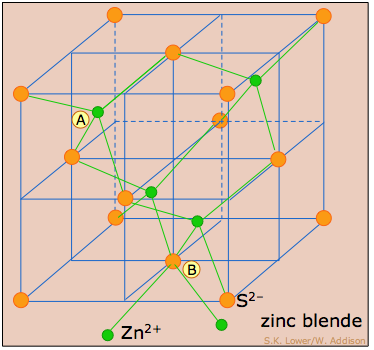
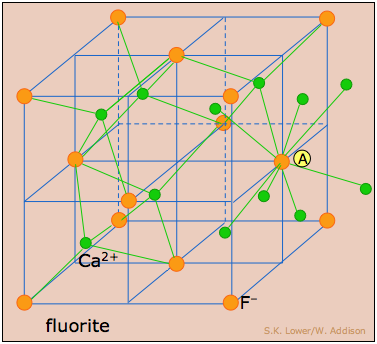
Cubic crystal lattices and close-packing
Page 1 of 1
 Cubic crystal lattices and close-packing
Cubic crystal lattices and close-packing
When substances form solids, they tend to pack together to form ordered arrays of atoms, ions, or molecules that we call crystals. Why does this order arise, and what kinds of arrangements are possible?
These are some of the questions we will explore in this lesson.
We will limit our discussion to cubic crystals, which form the simplest and most symmetric of all the lattice types. Cubic lattices are also very common — they are formed by many metallic crystals, and also by most of the alkali halides, several of which we will study as examples.
To continue clik below
[You must be registered and logged in to see this link.]
 Similar topics
Similar topics» Cubic Close Packing
» Tetrahedral and Octahedral Sites in Closest Packing
» Close Packed Structures
» Mechanism of the body-centered cubic--hexagonal close-packed phase transition in iron.
» Cubic Unit Cells and Their Origins
» Tetrahedral and Octahedral Sites in Closest Packing
» Close Packed Structures
» Mechanism of the body-centered cubic--hexagonal close-packed phase transition in iron.
» Cubic Unit Cells and Their Origins
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum